RESEARCH
Cellular stress responses are ancient, highly conserved programs that each of our cells can activate when they encounter harsh conditions. Turning on these responses powerfully rewires the central dogma, remodeling transcriptional, translational, and metabolic activity. When stress responses are activated at the right time and place they enable cellular survival until conditions improve—but chronic activation of stress responses is a hallmark of age-related and neurodegenerative diseases. Growing evidence suggests that chronic stress response signaling remodels cellular metabolism, but we lack a detailed understanding of the metabolic mechanisms underlying stress-linked diseases.
The decision to activate a stress response is coordinated by large protein machines that surveil cellular status and instigate the many diverse outcomes of stress response. Until recently, the mechanisms linking presence of a stressor to activation of the stress response remained murky. Key breakthroughs have been enabled by recent advances in structural biology: we can now visualize how conformational change within dynamic stress response machines encodes the stress responses. Importantly, this new structural understanding opens the door to rationally engineer any cell type of interest with specific adaptive or maladaptive stress response signaling states, enabling dissection of disease-relevant stress response mechanisms using classical biochemical and cell biological approaches.
Our lab is interested in questions like:
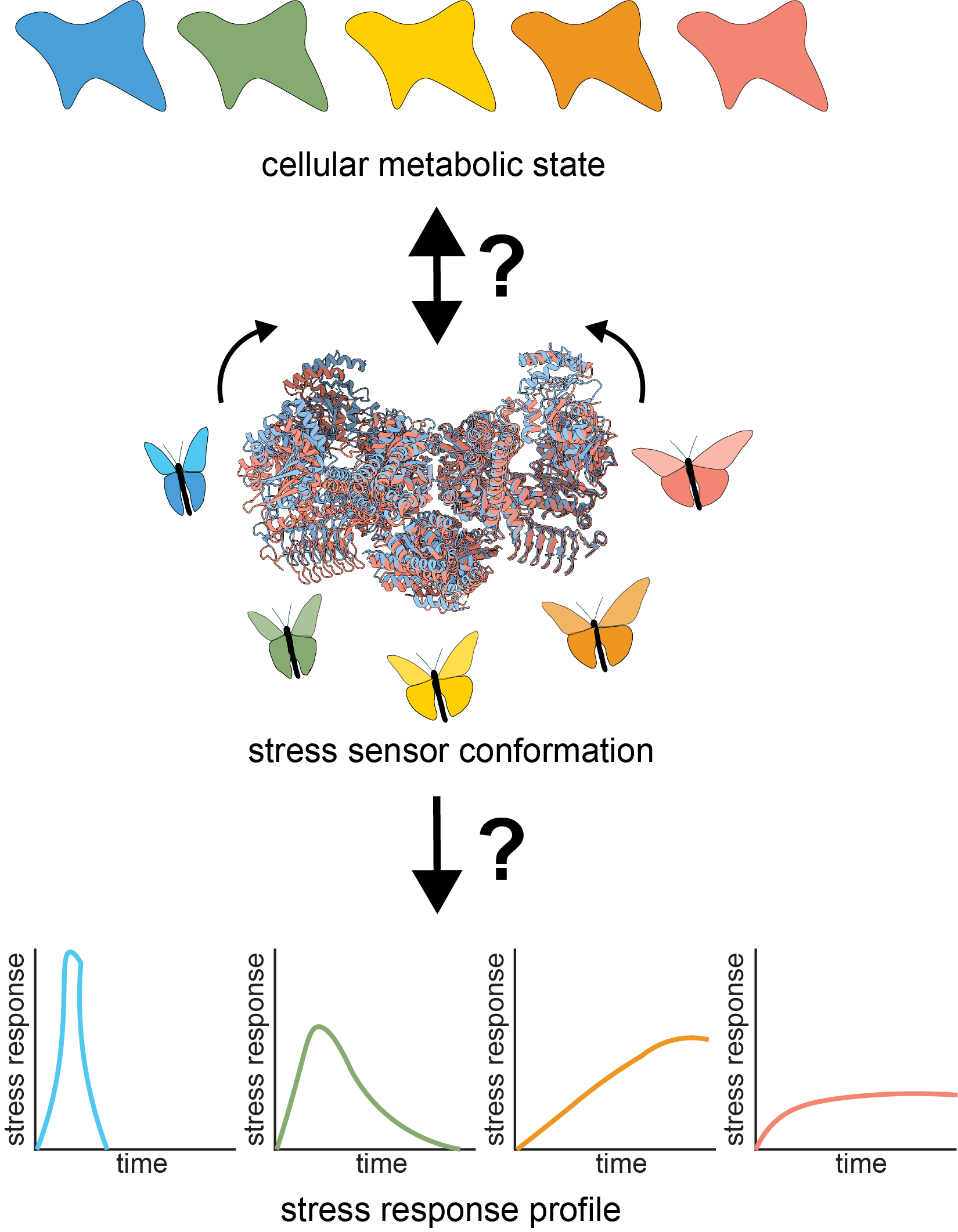
How do stress response machines sense and respond to cellular metabolic state?
What happens to cells whose stress response machines are chronically activated? How is their metabolism rewired?
How and why does chronic activation of stress response pathways impair specific cell types in the brain to drive neurodegenerative disease?
ON
OFF
ON
OFF
ONGOING PROJECTS
Mechanisms of cellular stress responsive decision-making
PROBLEM:
How does the stress response machinery sense cellular status and encode adaptive and maladaptive stress response states?
APPROACH:
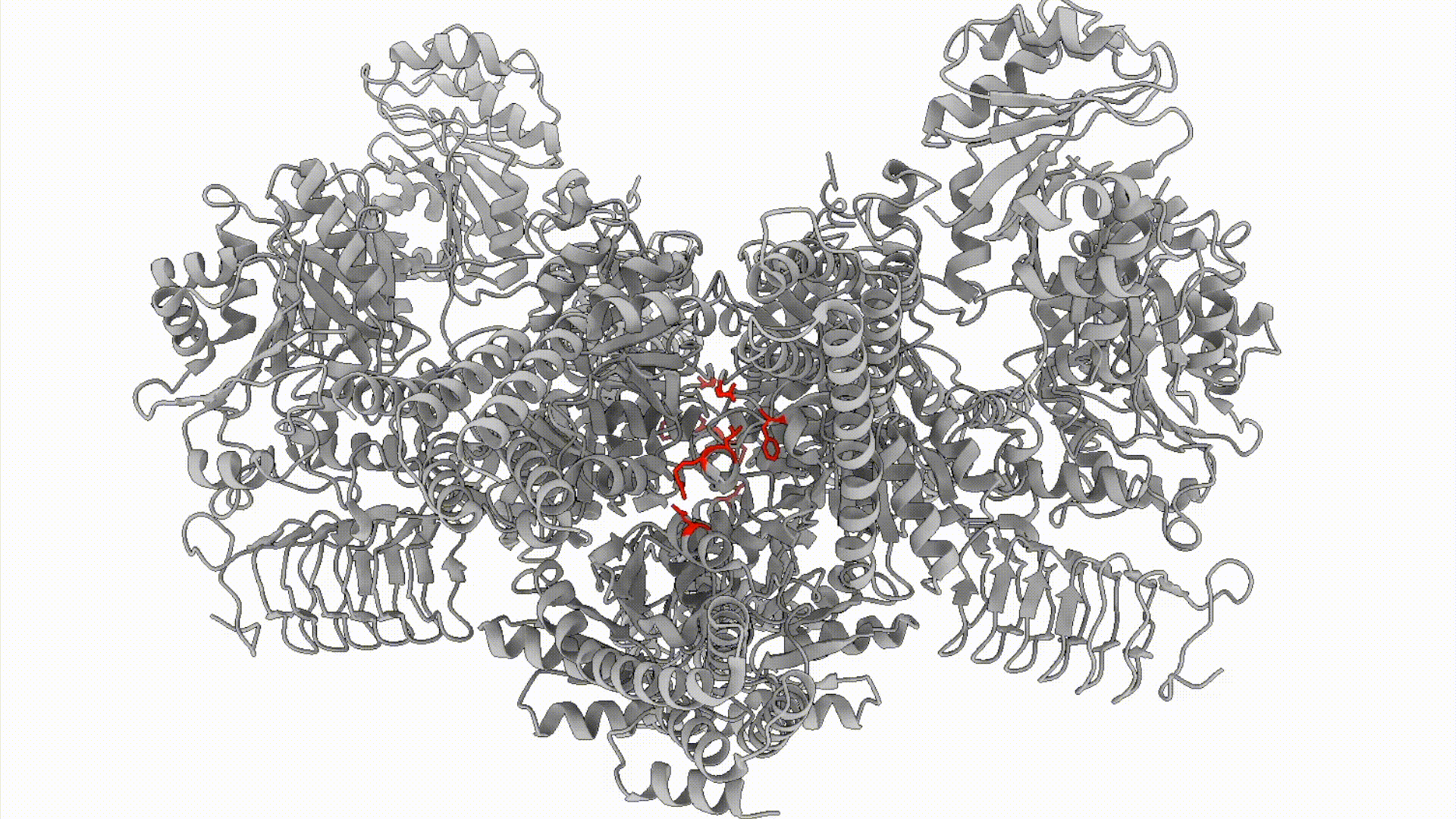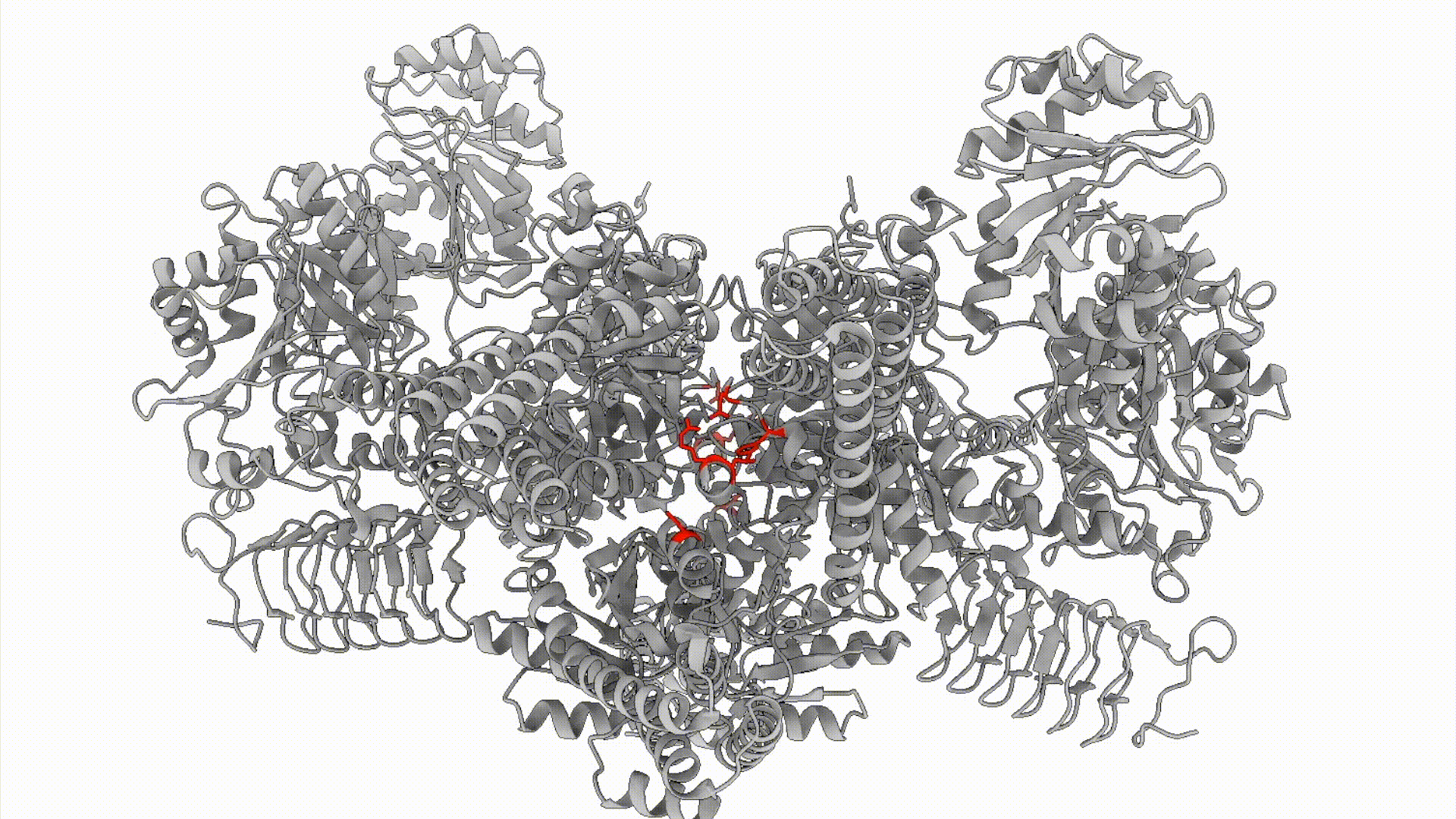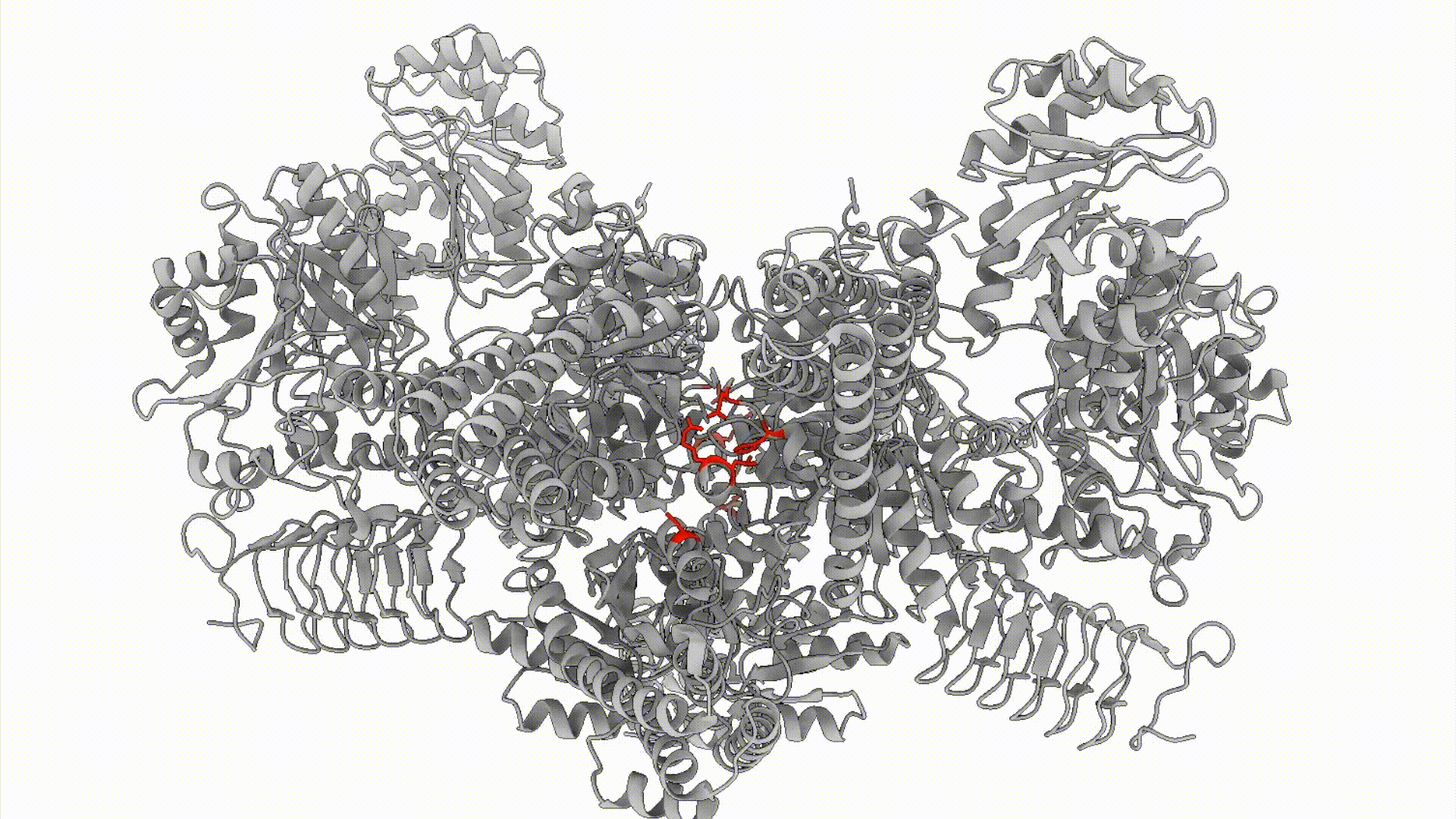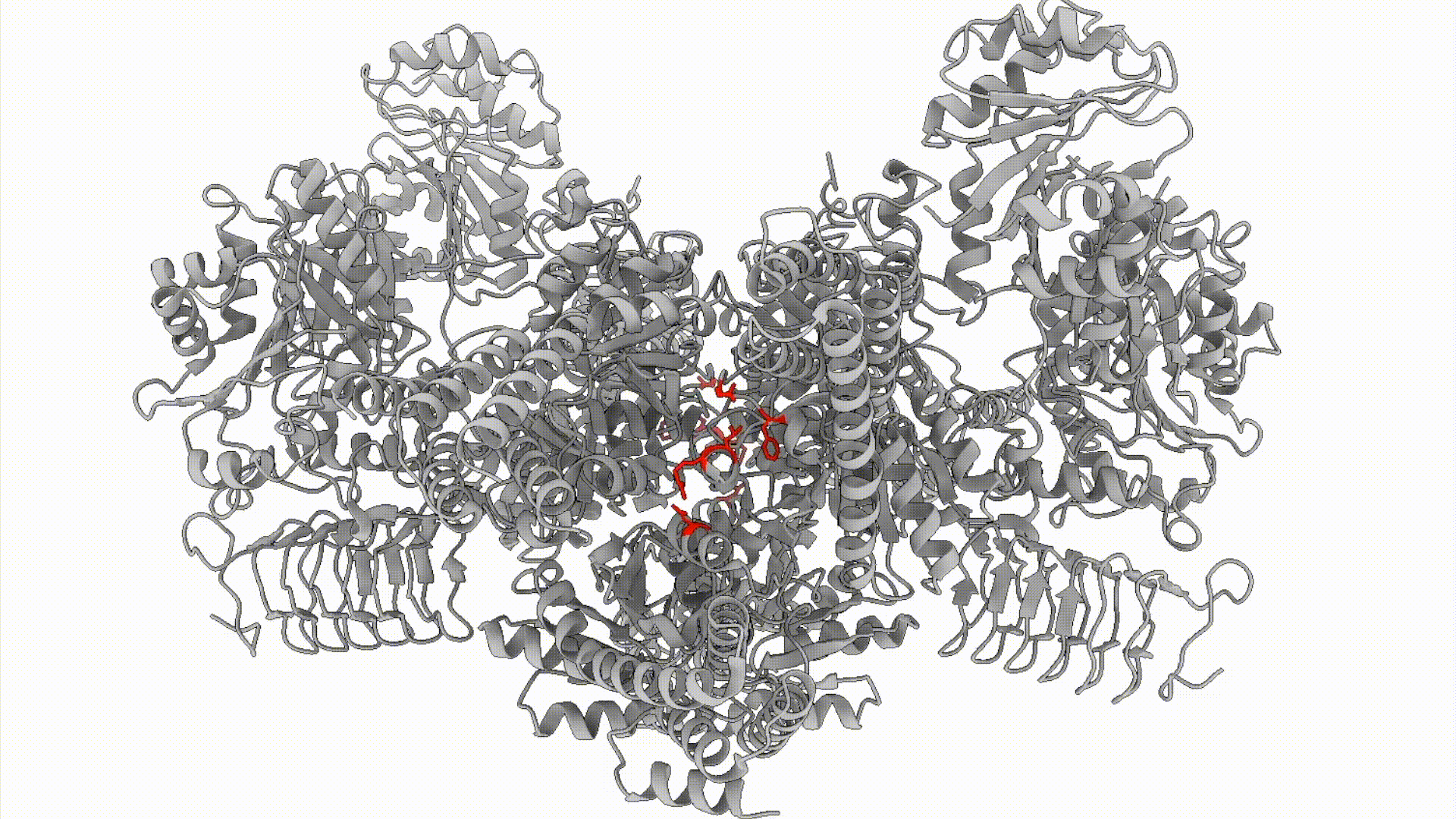
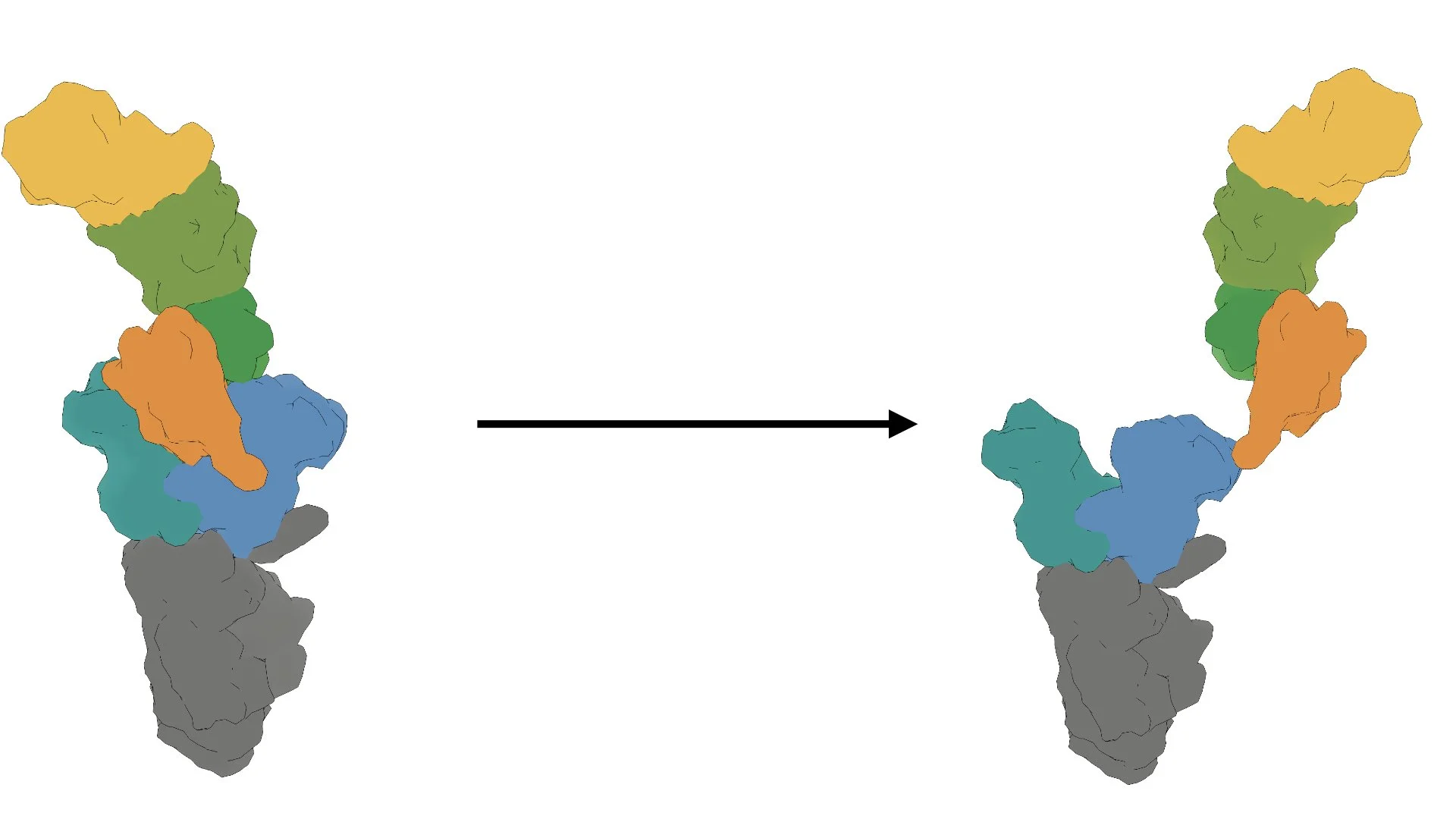
We combine biochemical, cell biological, and structural methods to visualize stress response decision-making in space and time. For example, we build off of recent work demonstrating that the Integrated Stress Response (ISR), is carried out by a conformational change (that resembles a butterfly flapping its wings) in the large, multimeric enzyme complex eIF2B. We are interested in questions like: How does eIF2B’s conformation dynamically response to cellular metabolic status? What metabolites can eIF2B sense?
Projects in this area can involve a combination of cellular biochemistry, cryo-EM, enzymology, and high-throughput methods including metabolomics and FLOW cytometry- based activity assays.
2. Mechanisms of selective vulnerability to chronic stress in glia
PROBLEM:
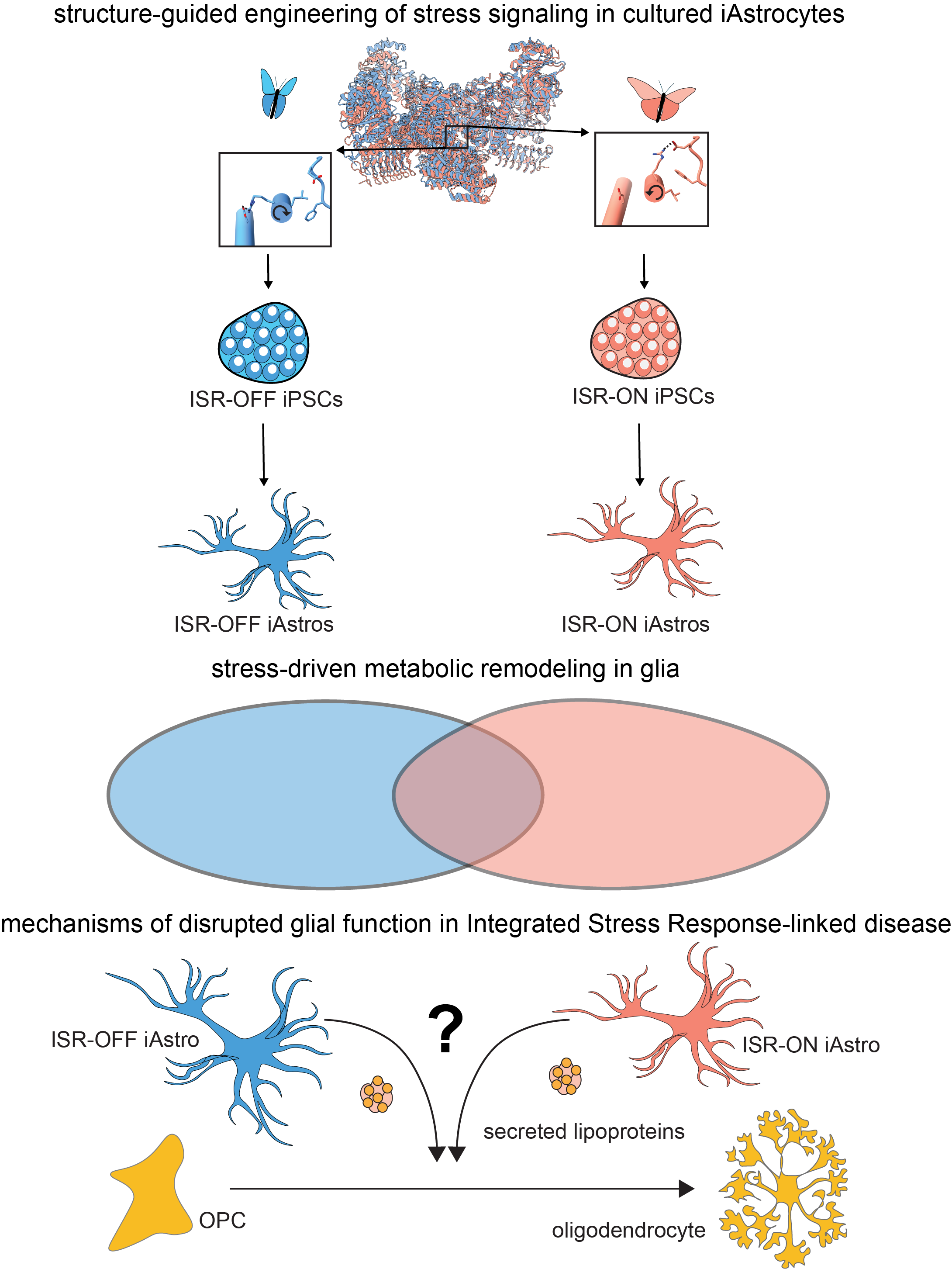
Cellular stress pathways are gradually upregulated during normal aging, and are hyperactivated in the context of age-related neurodegenerative disease. For example, chronic activation of the Integrated Stress Response (ISR) drives learning and memory defects in a wide range of neurodegenerative contexts, from Alzheimer’s disease to hypomyelinating disorders. But rather than affecting all cells equally, chronic ISR seems to selectively impair only certain highly specialized glial cell types in the brain.
How does chronic stress signaling selectively impair glial cell function?
APPROACH:
Mounting evidence suggests ISR signaling impairs glial function by remodeling cellular metabolism, but we have lacked the tools to define the underlying cellular mechanisms. We are using structure and AI-guided design to engineer novel chronic stress signaling iPSC-based cellular models, and use them as ideal biochemical systems in which to define how signaling is remodeled in response to stress.
Projects in this area combine biochemical, functional genomic (CRISPR), and cell biological methods to define mechanisms via which chronic ISR signaling impairs glial function in in vitro and in vivo models.